Dr. Daniel J. Leeman has either authored or reviewed and approved this content.
Page Updated:Breathe better, sleep better, feel better!
Practicing in Houston and The Woodlands, Texas, Sleep Apnea Specialist Dr. Shawn Allen is a Board-Certified Otolaryngologist (ENT) who understands that there is no “one size fits all” method for treating obstructive sleep apnea (OSA). To help each of his patients enjoy healthy, restful sleep, he offers a range of treatment options, including the revolutionary Inspire® Implant.

Sleep apnea is a sleep disorder characterized by interruptions in a patient’s airflow while they are sleeping. There are two reasons a person may experience interruptions or pauses in breathing while asleep:
Obstructive sleep apnea is the most common form of this sleep disorder, affecting between 10% and 30% of adults in the United States. Obstructive sleep apnea (OSA) can cause a range of symptoms, including:

In some patients with obstructive sleep apnea, airway obstruction is caused by a lack of muscle tone in the muscles at the back of the throat, which are stimulated by the
Trusted Source
Hypoglossal Nerve
Cleveland Clinic
Go to Source
hypoglossal nerve
. If these muscles are too relaxed, the tongue collapses over the larynx and obstructs the airway until the drop in oxygen level and rising heart rate trigger an awakening event that restores muscle tone.
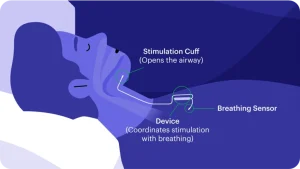
The Inspire hypoglossal nerve stimulator (HGNS) was approved by the FDA for the treatment of moderate to severe sleep apnea in 2014. Inspire is a small device similar in appearance to a pacemaker that is implanted over the muscles on the upper chest, usually on the right side of the body. A small incision in the neck on the same side is used to place an electrode around the portions of the hypoglossal nerve that open the airway. Once implanted, a sensor in the Inspire device identifies when a person is inhaling. Inspire then triggers a gentle pulse of electrical signals to stimulate the hypoglossal nerve, allowing the tongue to move slightly forward and opening the airway. After implantation with the Inspire device, patients simply use a remote control to turn it on at night and off in the morning
For most people who suffer from obstructive sleep apnea (OSA) a CPAP/APAP machine is the first line of treatment. CPAP machines worn during sleep provide continuous air pressure so that the airway remains open (unobstructed), and can be very effective in treating sleep apnea. However, the truth is that many patients are unable or unwilling to use their CPAP machine as they should. In fact,
Trusted Source
Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea
Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M
Go to Source
one study showed that up to 66%
of patients are not fully CPAP compliant.
Some reasons people don’t receive the full benefits of their CPAP include:
For qualified patients who struggle to use their CPAP effectively, find the CPAP to be intolerable or uncomfortable, or who wish to find a more permanent solution to sleep apnea, the Inspire hypoglossal nerve stimulator is a potential alternative.
Benefits of the Inspire Upper Airway Stimulation System include:
If you struggle with obstructive sleep apnea, Dr. Allen will conduct a thorough evaluation to determine whether the Inspire hypoglossal nerve stimulator implant may be the treatment for you. In general, candidates for the Inspire implant:
The first step in preparing for the Inspire implant is to undergo a sleep study to properly diagnose or confirm the severity of sleep apnea (results are generally considered adequate from prior testing if within 2 years from the date of Inspire surgery). Next, Dr. Allen will conduct a Drug-Induced Sleep Endoscopy (DISE) under IV sedation to evaluate the nature of your airway obstruction and confirm that Inspire therapy would adequately address your airway needs. Once Dr. Allen determines that you are a good candidate for sleep apnea treatment with the Inspire hypoglossal nerve stimulator implant, your procedure will be scheduled and you will be given detailed pre- and postoperative instructions.
Surgery to place the Inspire hypoglossal nerve stimulator is typically an outpatient procedure performed under general anesthesia. To begin the procedure, Dr. Allen will make two small incisions (generally right upper chest and right neck below the jawline). He will then place the battery-operated Inspire generator over the chest wall muscles and place the breathing sensor electrode against the muscles of the rib cage. The stimulation electrode is then applied to the hypoglossal nerve in the neck, where it is wrapped around the branches of the nerve that move the tongue forward. The device is fully tested and evaluated once in place. Once confirmed, Dr. Allen will close the incisions and the patient will be gently awakened from anesthesia and discharged to go home after a
Trusted Source
Upper Airway Stimulation/Hypoglossal Nerve Stimulator
American Thoracic Society
Go to Source
brief recovery period
.
The Inspire placement procedure takes between 2 and 3 hours on average, and you will be free to go home and rest after that. Some mild pain and swelling of the incision sites is to be expected, but most of Dr. Allen’s patients find adequate relief by taking over-the-counter medications. Dr. Allen will advise you to rest for the first few days after your procedure, and then you can resume most of your normal daily activities. You should wait a few weeks before engaging in more strenuous activities like working out or lifting your arm high above your head on the side of the incisions. Detailed instructions are given to avoid any confusion.

The Inspire hypoglossal nerve stimulator has been shown to be safe and effective by more than 90 peer-reviewed publications. According to some of these studies:
Yes. It is safe to walk through a metal detector with the Inspire HGNS. However, you should let the screening agent know that you have an implanted medical device.
It is safe to undergo X-rays, mammograms, and CT scans for patients who have the Inspire implant. Some models of the Inspire HGNS are not recommended for MRI (magnetic resonance imaging), so your doctor should contact Inspire Medical Systems or consult with your Patient ID card to determine eligibility. In general, the newer implant devices are MRI compatible but may limit visibility on MRI in areas of the body adjacent to the implant and electrodes.
Yes. Patients with the Inspire HGNS are not disqualified from having a pacemaker.

If you struggle with sleep apnea and have not been able to achieve the full benefits of using CPAP, you may be a good candidate for the Inspire hypoglossal nerve stimulation device. To learn more about Inspire, contact us to schedule a consultation with Dr. Allen, who is proud to serve patients from Houston, the Woodlands, and surrounding communities.
1 The American Sleep Apnea Association. What is Sleep Apnea? Available: https://www.sleepapnea.org/what-is-sleep-apnea/ Accessed October 3, 2022.
2 Cleveland Clinic. Hypoglossal Nerve. Available: https://my.clevelandclinic.org/health/body/21592-hypoglossal-nerve#:~:text=The%20hypoglossal%20nerve%20is%20one,and%20underside%20of%20your%20tongue. Accessed October 4, 2022.
3 Wolkove N, Baltzan M, Kamel H, Dabrusin R, Palayew M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can Respir J. 2008 Oct;15(7):365-9. doi: 10.1155/2008/534372. PMID: 18949106; PMCID: PMC2679572. Available: https://pubmed.ncbi.nlm.nih.gov/18949106/. Accessed October 4, 2022.
4 Food and Drug Administration. (2014, April 30). Summary of safety and effectiveness data (SSED). Available: https://www.accessdata.fda.gov/cdrh_docs/pdf13/P130008B.pdf. Accessed October 4, 2022.
5 American Thoracic Society. Upper Airway Stimulation/Hypoglossal Nerve Stimulator. Available at: https://www.thoracic.org/patients/patient-resources/resources/upper-airway-stimulation.pdf. Accessed October 4, 2022.
6 Woodson BT, Strohl KP, Soose RJ, Gillespie MB, Maurer JT, de Vries N, Padhya TA, Badr MS, Lin HS, Vanderveken OM, Mickelson S, Strollo PJ Jr. Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes. Otolaryngol Head Neck Surg. 2018 Jul;159(1):194-202. doi: 10.1177/0194599818762383. Epub 2018 Mar 27. PMID: 29582703. Available: https://pubmed.ncbi.nlm.nih.gov/29582703/. Accessed October 4, 2022.
7 Woodson BT, Strohl KP, Soose RJ, Gillespie MB, Maurer JT, de Vries N, Padhya TA, Badr MS, Lin HS, Vanderveken OM, Mickelson S, Strollo PJ Jr. Upper Airway Stimulation for Obstructive Sleep Apnea: 5-Year Outcomes. Otolaryngol Head Neck Surg. 2018 Jul;159(1):194-202. doi: 10.1177/0194599818762383. Epub 2018 Mar 27. PMID: 29582703. Available: https://pubmed.ncbi.nlm.nih.gov/29582703/. Accessed October 4, 2022.
8 Heiser C, Steffen A, Boon M, Hofauer B, Doghramji K, Maurer JT, Sommer JU, Soose R, Strollo PJ Jr, Schwab R, Thaler E, Withrow K, Kominsky A, Larsen C, Kezirian EJ, Hsia J, Chia S, Harwick J, Strohl K, Mehra R; ADHERE registry investigators. Post-approval upper airway stimulation predictors of treatment effectiveness in the ADHERE registry. Eur Respir J. 2019 Jan 3;53(1):1801405. doi: 10.1183/13993003.01405-2018. PMID: 30487205; PMCID: PMC6319796. Available: https://pubmed.ncbi.nlm.nih.gov/30487205/. Accessed October 4, 2022.
9 Heiser C, Steffen A, Boon M, Hofauer B, Doghramji K, Maurer JT, Sommer JU, Soose R, Strollo PJ Jr, Schwab R, Thaler E, Withrow K, Kominsky A, Larsen C, Kezirian EJ, Hsia J, Chia S, Harwick J, Strohl K, Mehra R; ADHERE registry investigators. Post-approval upper airway stimulation predictors of treatment effectiveness in the ADHERE registry. Eur Respir J. 2019 Jan 3;53(1):1801405. doi: 10.1183/13993003.01405-2018. PMID: 30487205; PMCID: PMC6319796. Available: https://pubmed.ncbi.nlm.nih.gov/30487205/. Accessed October 4, 2022.
Dr. Daniel J. Leeman has either authored or reviewed and approved this content.
Page Updated: